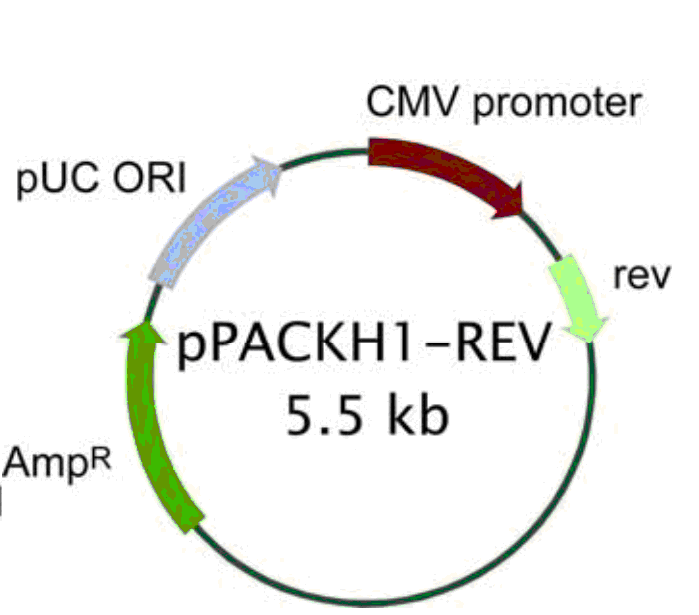
pPACKH1-REV
pPACKH1-REV
编号 | 载体名称 |
北京华越洋VECT231282 | pPACKH1-REV |
pPACKH1-REV载体基本信息:
载体名称: | pPACKH1-REV |
质粒类型: | 慢病毒包装载体 |
高拷贝/低拷贝: | 高拷贝 |
克隆方法: | 限制性内切酶,多克隆位点 |
启动子: | CMV |
载体大小: | 5.5kb |
5' 测序引物及序列: | CMV Forward: CGCAAATGGGCGGTAGGCGTG |
3' 测序引物及序列: | -- |
载体标签: | -- |
载体抗性: | 氨苄青霉素 |
筛选标记: | -- |
克隆菌株: | TOP10等常规菌株 |
宿主细胞(系): | 哺乳动物细胞系 |
备注: |
第三代慢毒包装载体pPACKH1-GAG与pPACKH1-REV, pVSV-G是基于HIV-1的慢病毒包装系统。
用于第三代慢病毒载体的包装,如: pCDH-CMV-MCS-EF1-puro pCDH-CMV-MCS-EF1-copGFP pCDH-CMV-MCS-EF1-copGFP-T2A-puro pCDH-CMV-MCS-EF1-RFP pCDH-MCS-T2A-puro-MSCV |
稳定性: | 瞬表达 |
组成型/诱导型: | 组成型 |
病毒/非病毒: | 非病毒 |
pPACKH1-REV载体质粒图谱和多克隆位点信息:
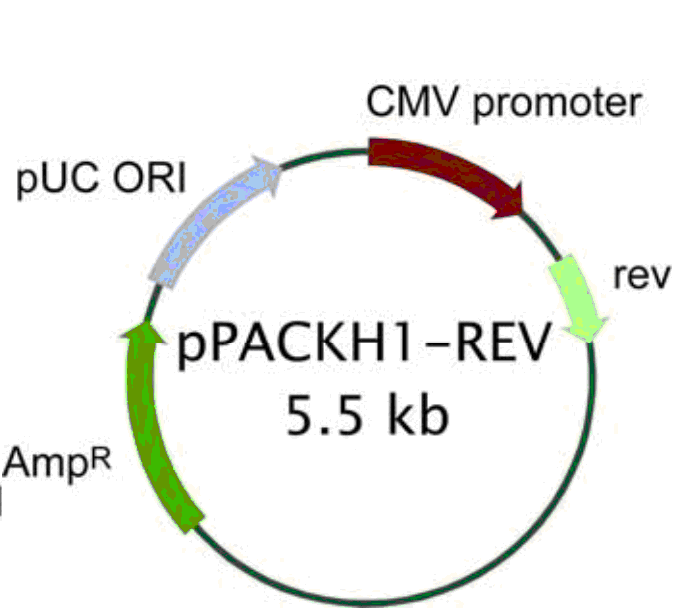
pPACKH1-REV载体简介:
慢病毒包装质粒pPACKH1-REV使用方法——慢病毒包装与转染方法
Production of lentiviral viral stocks requires HIV-1 gag, pol, and rev gene products and vesicular stomatitis virus G (VSV-G) protein encoded by helper plasmids. In general, at least two helper plasmids are required, with one plasmid expressing the Gag-Pol polyprotein and an accessory protein Rev and the other expressing VSV-G as envelop protein to increase cell tropism. Nevertheless, Gag-Pol and Rev proteins can be expressed from separated plasmids as well. Therefore, a three-plasmid system or a four-plasmid system can be used to generate viral stocks, depending on the source and nature of the helper plasmids. In the three-plasmid system, lentiviral transfer vector is cotransfected with two helper plasmids (Gag-Pol + Rev and VSV-G) into cells, while in the four-plasmid system; lentiviral transfer vector is cotransfected with three helper plasmids (Gag-Pol, Rev and VSV-G). It is generally considered to be safer to produce the lentiviral stocks with more helper plasmids, due to the reduced chance of recombination among all vectors that generates replication-competent viruses. The manufacturers of transfection reagents, the suppliers of lentiviral packaging constructs, and many academic laboratories have provided protocols for producing lentiviral stocks. The following procedure is provided as an example only. We produce lentiviral stocks in 293T cells using the transfection conditions summarized in a table below.
10-cm plate | 6-well plate | |||
3-plasmid system | 4-plasmid system | 3-plasmid system | 4-plasmid system | |
Lentiviral vector | 9 μg | 1.5 μg | ||
Gag-Pol + Rev expression vector1 | 6 μg | 1.0 μg | ||
Gag-Pol expression vector2 | 4.5 μg | 0.75 μg | ||
Rev expression vector3 | 1.8 μg | 0.3 μg | ||
VSV-G expression vector4 | 3 μg | 2.7 μg | 0.5 μg | 0.45 μg |
Total plasmid DNA | 18 μg | 3.0 μg | ||
Lipofectamine™ 2000 | 45 μl | 7.5 μl | ||
Total Opti-MEM | 3 ml | 0.5 ml | ||
293T cells / vol. of medium | 1.0 × 107/5ml | 1.7 × 106/1ml | ||
Below we have listed the commonly used vectors for lentiviral packaging.
1 For example: pCMV-deltaR8.91 (TRC), psPAX2 (Addgene)
2 For example: pMDLg/pRRE (Addgene), pLP1 (Invitrogen), pPACKH1-GAG (SBI)
3 For example: pRSV-REV (Addgene), pLP2 (Invitrogen), pPACKH1-REV (SBI)
4 For example: pMD.G (TRC), pMD2.G (Addgene), pCMV-VSV-G (Addgene), pVSV-G (SBI), pLP/VSVG (Invitrogen)
Note: The transfection reagent Lipofectamine™ 2000 (LF2000, Invitrogen) is preferred for transfection. The average lentiviral titers in our preparations are around 5 x 106 - 5 x 107 infection units per ml (IU/ml) when titered with 293T cells.
Day 0: Seed 6.0 × 106 (1.0 × 106) 293T cells in a 10-cm plate (6-well plate), so that the cell density will be around 1.0 × 107 (1.7 × 106) at the time of transduction.
Day 1: Gently mix 45.0 (7.5) μl LF2000 and 1.5 (0.25) ml Opti-MEM medium and incubate at room temperature for 5 minutes. Meanwhile, gently mix 18.0 (3.0) μg in total of lentiviral transfer vector and helper plasmids mixture into 1.5 (0.25) ml Opti-MEM medium (Invitrogen).
Gently mix DNA and LF2000, incubate at room temperature for 20 minutes to allow DNA and lipid to form complexes. In the meantime, replace the overnight culture medium with 5.0 (1.0) ml DMEM + 10% FBS without antibiotics. Add the 3.0 (0.5) ml DNA-LF2000 complexes to 293T cells.
Note: We have noticed that fetal bovine serum purchased from different manufacturers may affect the attachment of 293T cells to the bottom of tissue culture plates, resulting in variation in the efficiency of lentiviral production. We recommend switching to a different brand of FBS if 293T cells disattach from plates during lentiviral production.
Day 2: Replace the media containing the DNA-LF2000 complexes with 10.0 (2.0) ml complete medium at 12-16 hours post-transfection.
Day4: Collect supernatants at 48 hours post-transfection and transfer media to a polypropylene storage tube. Spin the virus-containing media at 1300 rpm for 5 minutes to pellet any 293T cells that were carried over during collection. Carefully transfer the supernatant to a sterile polypropylene storage tube.
Note: Lentiviral stock may be stored at 4 °C for up to 5 days, but should be aliquoted and frozen at -80 °C for long-term storage.
Suggestion: To reduce the number of freeze and thaw cycles, aliquot lentiviral stock to smaller tubes before storage at -80 °C.
Titering the lentiviral stocks
It is important to titer the lentiviral stocks in the cell line of interest to produce consistent results using the equivalent multiplicity of infection (MOI) in experiments. Knowing MOI will help you to control the copy number of lenti-cDNA integrated into the chromosomes of the cells of interest. While titering virus with antibiotic selection, the titering procedure includes selection of stably transduced cells with the corresponding antibiotics and counting the antibiotic-resistant cell colonies. Alternatively, a flow cytometry can be used to determine the viral titer by measuring the number of green (or red) fluorescent cells. If you are using both methods to titer your lentiviruses, please keep in mind that the titers may be different due to sensitivities of FACS machine and cell lines resistant to drug selection.
Please be aware the lenti-cDNA virus titer may be underestimated when antibiotic selection or flow cytometry is deployed to determine the titer. For example, the transduced cells with single integration event may not be selected due to low level of selection marker expression by IRES.
A. Determination of lentiviral titers by antibiotics selection.
Day 0: Seed the cells of your choice in a 6-well plate so that the cell density that will be ~25-50% confluent at the time of transduction.
Day 1: Thaw lentiviral stock, gently mix virus and then prepare 2 ml 10-fold serial dilutions ranging from 10-3 to 10-7 in complete medium containing 5-8 μg/ml polybrene.
Remove the medium from previous day and add 2 ml fresh dilutions into each well.
Suggestion: Leave one well uninfected for mock control. Spin transduction in a desktop centrifuge (e.g. Sorvall RT6000) at 1,000 × g for 30-60 min at room temperature may be necessary if titering virus in cell lines other than 293T.
Day 2: Replace the medium containing virus and polybrene with 2 ml of complete medium.
Day 3-4: Replace medium with fresh medium containing antibiotic to select for stably transduced cells.
Note: At least 48 hours of transduction allows lenti-cDNA to integrate into the host genome. The optimal antibiotic concentration varies from cell line to cell line.
Suggestion: A pilot experiment should be performed to determine the minimum concentration of antibiotics required to kill the untransduced cells before this experiment.
Day 5-6: Replace medium with 2 ml fresh medium containing antibiotic every 2 days.
Day 7-8: Allow antibiotic-resistant colonies to form in dilution wells. No live cells should be growing in the mock control well.
Note: The number of days required for the formation of visible colonies may vary among different cell lines.
Wash wells twice with 2 ml PBS
Stain cells with 1 ml 0.5% crystal violet solution in 20% ethanol and incubate for 30 minutes at room temperature.
Wash wells with distilled water by submerging the plate in a tray full of water, and repeat the wash one more time.
Dry the plate and count the number of blue-stained colonies.
The titer should be the average colony number times the dilution factor.
Note: The transduction efficiency varies from cell line to cell line. The lentiviral titer in the cell line of your interest may be lower (sometimes more than 10-fold) or higher than the virus titer in commonly used cell lines such as 293T cells.
B. Determination of lentiviral titers by flow cytometry.
Day 0: Seed the cell of your choice in a 6-well plate so that the cell density that will be ~25-50% confluent at the time of transduction.
Note: The number of cells seeded in the well is required to calculate lentiviral titer later.
Day 1: Thaw lentiviral stock, gently mix virus and then prepare 2 ml 10-fold serial dilutions ranging from 10-1 to 10-4 in complete medium containing 5-8 μg/ml polybrene.
Remove the medium from previous day and add 2 ml fresh dilutions into each well.
Suggestion: Leave one well uninfected for mock control. Spin transduction in a desktop centrifuge (e.g. Sorvall RT6000) at 1,000 × g for 30-60 min at room temperature may be necessary if titering virus in cell lines other than 293T.
Day 2: Replace the medium containing virus and polybrene with 2 ml of complete medium.
Day 3-4: Follow your lab protocol to collect and resuspend cells for flow cytometry to determine the percentage of green or red fluorescent cells.
Note: At least 48 hours of transduction allows lenti-cDNA to integrate into the host genome.
The titer should be the average number of live fluorescent cells times the dilution factor.
Note: The transduction efficiency varies from cell line to cell line. The lentiviral titer in the cell line of your interest may be lower (sometimes more than 10-fold) or higher than the virus titer in commonly used cell lines such as 293T cells.
Example: 1 × 105 cells were seeded on Day 0 and the cell doubling time is around 24 hours. The FACS data showed 5% and 40% of green fluorescence cells in the 10-3 and 10-2 dilution wells, respectively. The lentiviral titer is calculated by multiplying the fraction of transduced cells by 2 × 105 (the expected number of cells in the well on Day 1, the time of transduction), and by the dilution factor.
0.05 × (2 × 105) × 103 = 1 × 107
0.4 × (2 × 105) × 102 = 8 × 106
The average lentiviral titer is 9 × 106 IU/ml
Lentiviral Transduction for Gene Expression.
Day 0: Seed cells at appropriate density.
Suggestion: Plate cells so that cell density will be ~10-25% confluent at the time of transduction.
Day 1: Transduction. Remove the medium from the tissue culture plate by aspiration and replace it with fresh complete medium containing 5-8 μg/ml polybrene. Gently mix lentivirus with pipette tip, and add appropriate amount of virus to each well.
Note: (1) Polybrene may be toxic to some cell lines. (2) The non-concentrated and non-purified lentiviral stock collected from 293T supernatant may contain substances affecting the target cell growth, especially when a large volume of low-titer lentiviral stock is added. In case the lentiviral stock inhibits cell growth, concentration and purification of the viruses may be required. (See below for a protocol of virus concentration) Alternatively, incubation time for transduction can be shortened to hours. For example, the virus-containing medium may be replaced with fresh medium after one hour of transduction.
Suggestion: Transduce cells at multiplicity of infection (MOI) = 1, 5, 10, 25, and 50 to determine the optimal expression level. Spin transduction in a desktop centrifuge (e.g. Sorvall RT6000) at 1,000 × g for 30-60 min at room temperature helps increase of transduction efficiency.
Day 2: Replace the transduction medium with fresh complete medium to remove lentivirus and polybrene.
Day 3-4: Select transduced cells (>50% confluence is recommended) with medium containing appropriate antibiotics or by flow cytometry to sort out fluorescence-positive cells if necessary.
Note: The optimal antibiotic concentration varies from cell line to cell line.
Suggestion: A pilot experiment should be performed to determine the minimum concentration of antibiotic required to kill the untransduced cells before this experiment.
Day 6+: Analysis of transduced cells.
Suggestion: Expand the culture of cell lines stably expressing GOI and store the cell line stocks in liquid nitrogen before analyzing the cells.
Optional: Concentration of lentivirus by ultracentrifugation
1. Filter lentivirus through a 0.45 μm filter.
2. Centrifuge at 25,000 rpm for 90 minutes in a SW-28 or SW-41 rotor.
3. Discard the supernatant and use a Pasteur pipette with an attached P100 tip to carefully remove the remaining medium.
4. Gently resuspend viral pellet in 1/100 volume of DMEM. Let the virus suspension sit for overnight at 4° C.
5. On the following day, mix gently, aliquot and freeze virus.
pPACKH1-REV载体序列
pPACKH1-REV其他相关慢病毒载体: